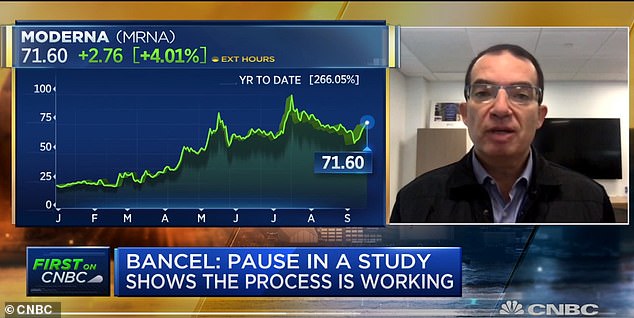
Thursday, the CEO of American company Moderna said it expects to know whether its coronavirus vaccine works by November, based on its latest trial still in progress. IF they can demonstrate that the vaccine is 70% effective they will petition the FDA to give emergency authorization so it can be administered to frontline workers and the most at risk. To get this kind of clearance a vaccine must demonstrate they are ‘at least 50 percent more effective than a placebo to be considered for approval. ‘
One of six companies racing to find a vaccine, this is the most promising.
So, some good news.